Kathryn Jenkins
Our laboratory automated stainers use an alcohol-based stain, which provides enhanced stain quality and consistency when staining large numbers of cytology smears and blood films. Compared to quick (aqueous based) stains, alcohol-based stains (such as modified Wrights) allow improved characterisation of nuclear detail, and increased detection of mast cells (especially poorly differentiated cells), granular lymphocytes, grey eosinophils, and less common diseases including lysosomal storage disease.
Importantly, the automated stainers have a specific range of staining (central 2/3 of the glass slide). This means that samples outside this area will remain unstained. Cases can then be re-stained with a quick stain to capture the ends, however these areas are then subject to the limitations as mentioned above. Additionally, material at the edges of the slides can be very difficult to examine at higher power due to the physical limitations of the microscope stage.
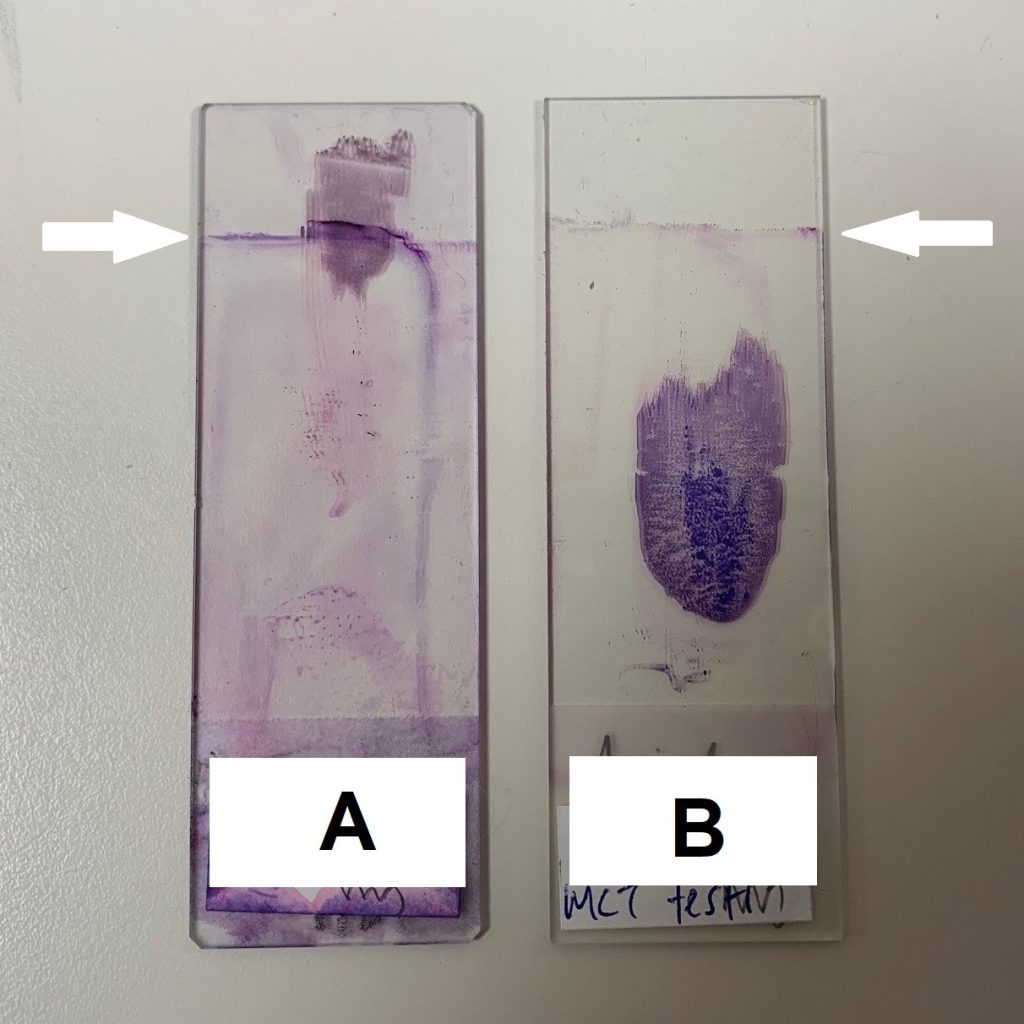
Help us to help you, by ensuring blood films and cytology samples are located within the central 2/3 of the glass slide (see figure 1). Slide A shows a sample at the end of the glass slide, which needed to be re-stained using a quick stain. Slide B shows a sample in an ideal central location. The stain demarcation line can be clearly seen (arrow).
Figure 1 (below). Slide A—the sample is placed too close to the edge of the slide and most of the material is not able to be stained by the automatic stainer. Manual re-staining was required. Note the line (arrow) where the automated stainer does not reach. Slide B—sample is in centre of slide and has been completely stained.