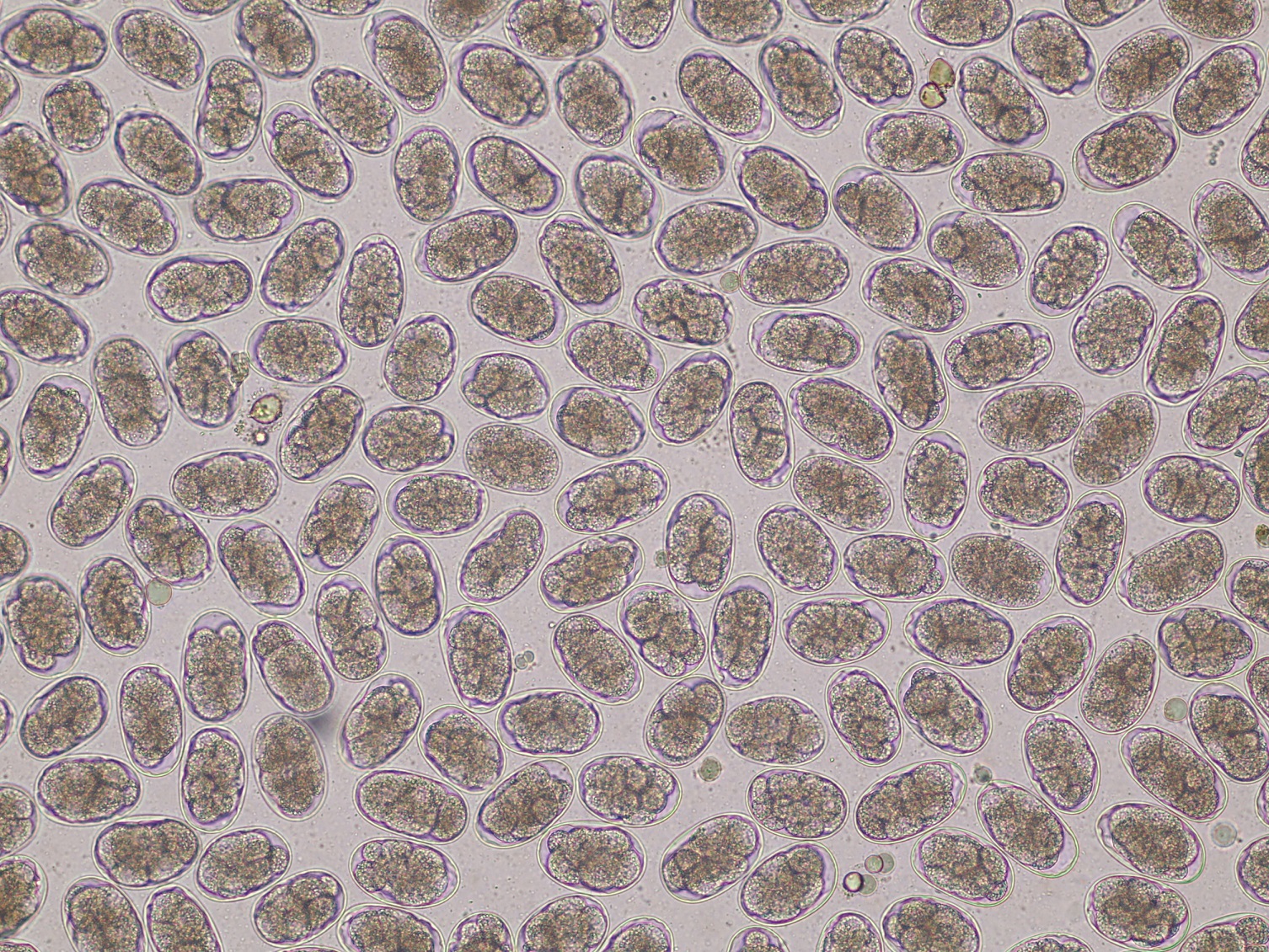
Species: Cattle, sheep
Specimen: 2-4g faeces per animal
Container: Plastic pottle
Collection protocol:
Collect samples directly from rectum of animal (not from the ground unless freshly passed onto clean yard). Sample from 10-15 animals per group. Equal volumes of faeces may be pre-bulked or sent individually.
Special handling/shipping requirements:
Submit fresh or store in a refrigerator* until transport to a laboratory. Do not freeze.
*It is important that faecal samples for larval cultures are not refrigerated at all. In cases where both FECs and larval cultures are required on the same set of samples it is recommended that a sub-sample be removed from each individual sample and pooled. The individual samples for FEC can be refrigerated while the pooled sample should be clearly identified as being for larval culture and kept at room temperature.
General information about the disease:
Grazing ruminants in New Zealand are rarely free of worm infection, though effects on stock health and productivity vary widely. Clinical effects of enteric parasitism include ill thrift, diarrhoea, anaemia, and death in severe cases. The degree of damage is influenced by the numbers and identities of the parasites present, host age, immunity, general health, and nutrition.
General information about when the test is indicated:
Composite FECs are used to give an overview of the gastrointestinal nematode parasite status of a flock or herd. Indications include monitoring the effectiveness of control programmes, assisting in drench decision-making, and helping to identify the causes of scouring and wasting. They can also be used as a common pre-treatment control group for faecal egg count reduction tests.
Comparison with other related tests:
FECs provide little information on the identity of the worm genera represented. This can be overcome by the use of faecal larval cultures in conjunction with FEC. FEC and larval culture are both necessary for the calculation of faecal egg count reductions to determine drench resistance. Total worm counts on abomasal and small intestinal contents can provide more accurate information on parasite burden than FECs. Serum pepsinogen may be used as an estimate of abomasal ostertagiasis in young cattle.