SUNAO FUJITA
Immunocytochemistry (ICC) is an adjunct diagnostic tool, which helps to identify cellular origin utilizing the accuracy of antibody-antigen binding. In comparison with immunohistochemistry (IHC), which is more commonly used in both human and veterinary medicine, ICC is not routinely exploited for the classification of a variety of neoplastic lesions.
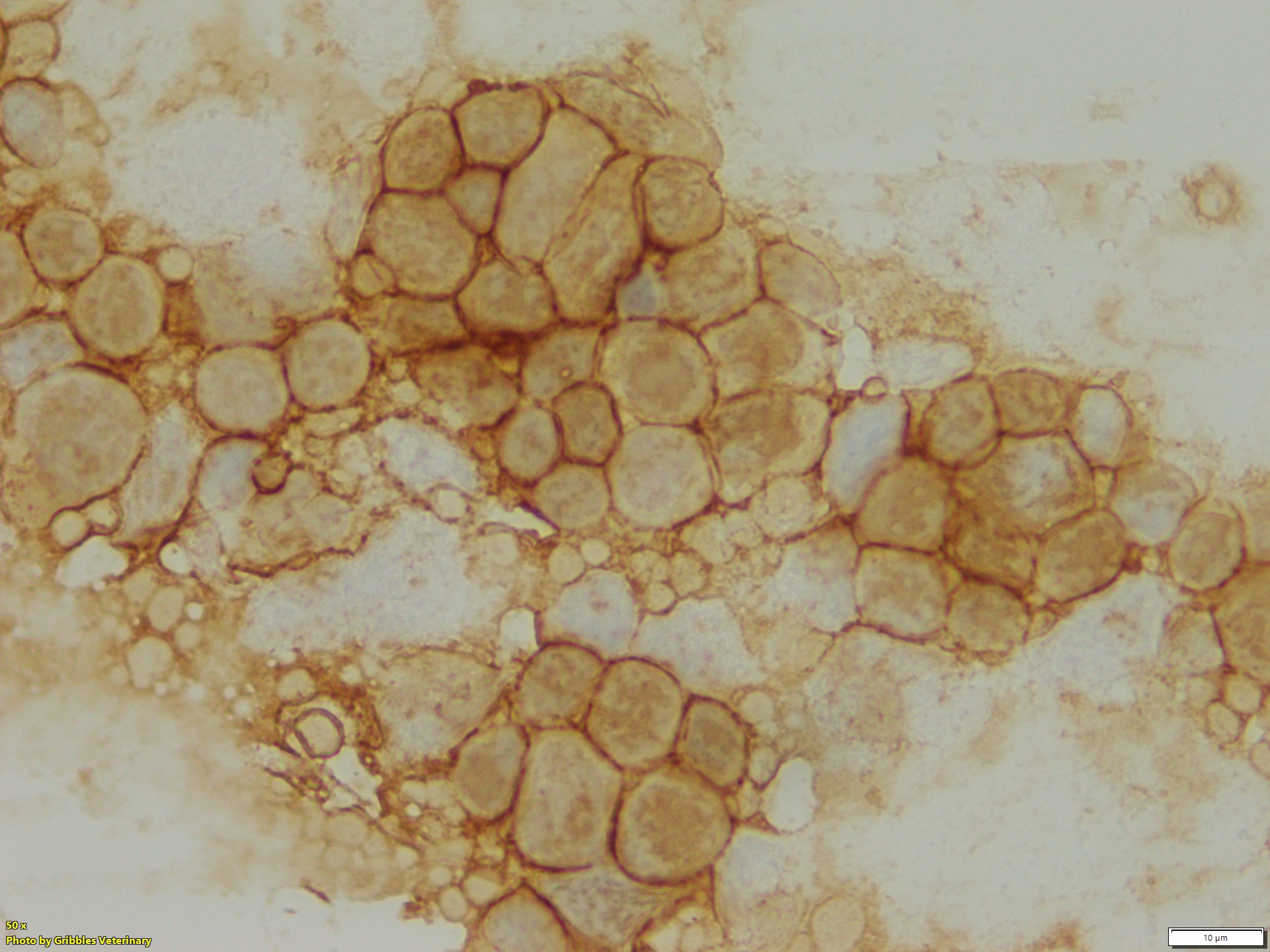
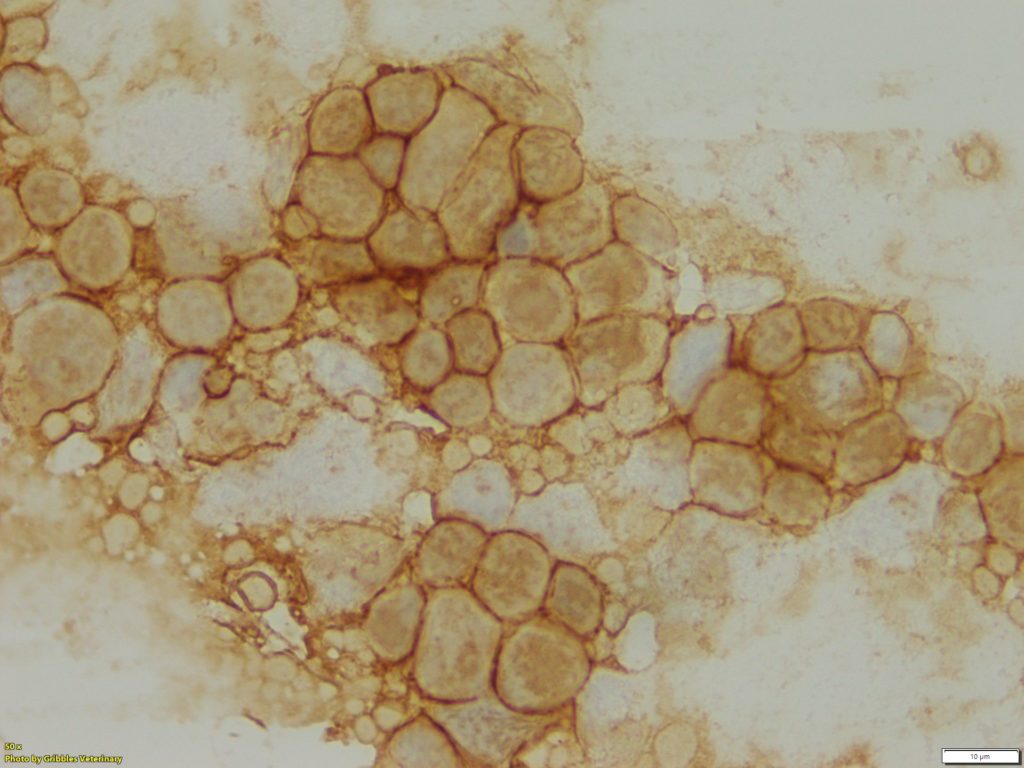
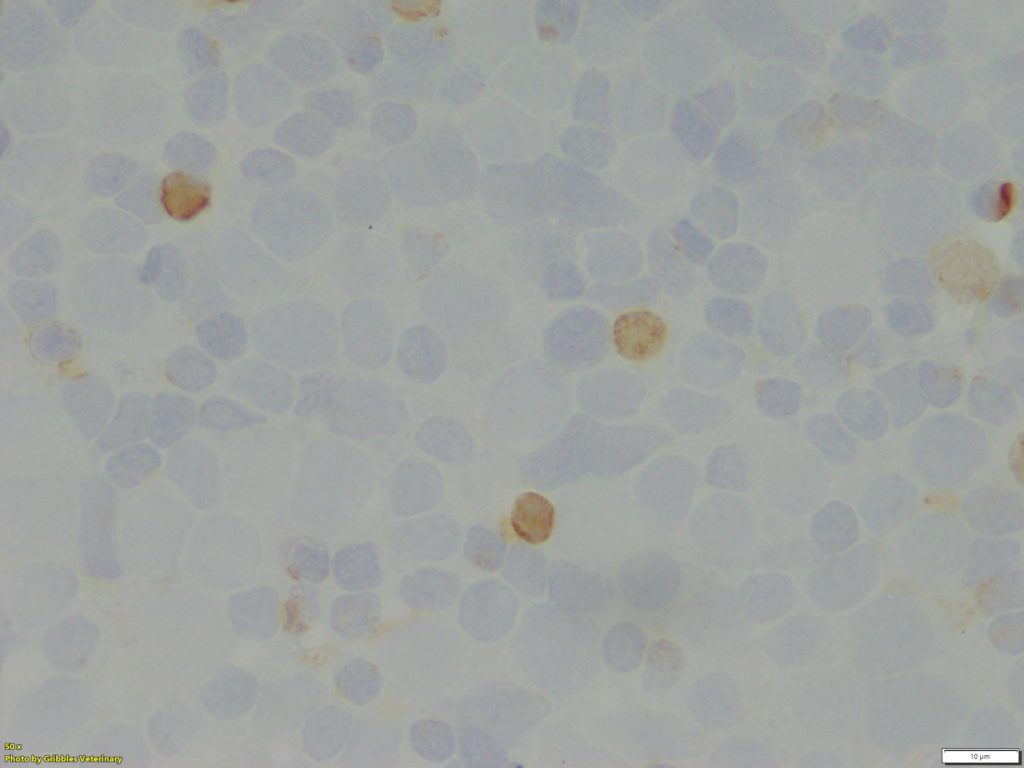
However, ICC has the potential to be a valuable diagnostic tool, not only to identify cellular origin, but also to obtain prognostic information about neoplastic diseases. As of now, ICC is primarily used for immunophenotyping of lymphoid neoplasms (lymphoma and lymphocytic leukaemia) in veterinary medicine, especially classification of B- or T-cell neoplasms. While IHC remains the gold standard for immunophenotyping of lymphoid neoplasia, excellent correlation between ICC and IHC has been reported in publications1. Gribbles offers two lymphoid cell markers for samples from dogs, CD20 for B-cells and CD3 for T-cells.
The majority of lymphomas in dogs are not challenging to diagnose on routine cytology samples, particularly on aspirates of peripheral lymph nodes. However, immunophenotype and evaluation of prognosis cannot be determined from the cytology alone. Immunophenotyping is one of the crucial elements for classification of canine lymphoma. Treatment plans (chemotherapy protocol) and prognosis can vary depending on the ICC results. Sometimes, round cell neoplasm might be difficult to distinguish by morphology alone. Utilizing the lymphoid cell markers, CD3 and CD20, can be beneficial for confirmation of lymphoid origin.
ICC is used to immunophenotype lymphoid neoplasms.
Basically, ICC is not an option to confirm the diagnosis of lymphoma in suspect or nebulous cases. It is NOT a test to differentiate lymphoma from a normal or hyperplastic lymphoid population on lymph node cytology samples. ICC is only an adjunct test to provide immunophenotype information.
Furthermore, definitive subtyping requires evaluation of tissue architecture by means of histopathology in addition to immunophenotyping (e.g. indolent lymphoma).
Advantages of ICC
> Minimally invasive and low cost of sampling procedure – use of FNA sample
> Low anaesthetic risk
> Easy to re-sample if non-diagnostic results obtained
> Can quickly provide pre-surgical diagnosis in cases requiring surgical treatment.
Limitations of ICC
> Significant variation in cytology slide quality because of using FNA slides
> Multiple identical slides are not available
> Not helpful to confirm a neoplastic proliferation
Samples
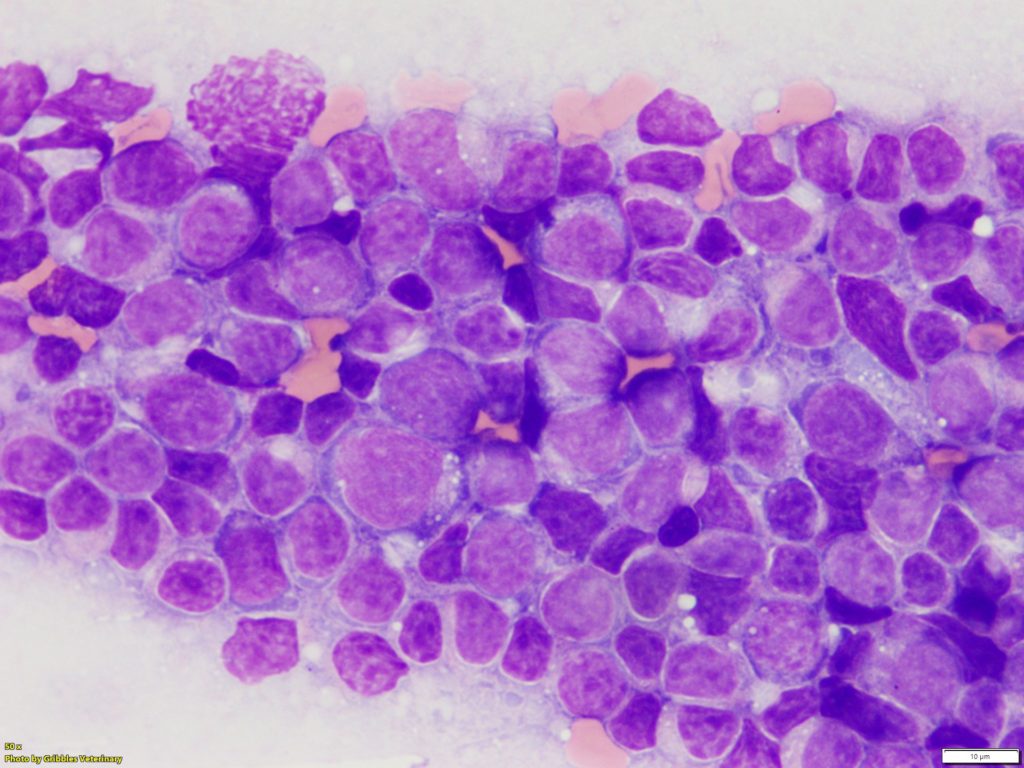
At Gribbles Veterinary, the samples we usually receive for ICC are direct smears of FNAs taken from enlarged lymph nodes . FNA samples are very easy to obtain, but their sample quality varies quite a bit depending on sampling technique.
Lymphocytes are inherently fragile and are easily ruptured, resulting in lack of adequate numbers of intact cells and abundant cytoplasmic fragments in a background of smears. Additionally, inadequate cellularity can also be a problem (sometimes, no lymphoid cells are harvested). These issues can cause difficulties with interpretation of ICC slides.
Therefore, we perform routine staining (Wright Giemsa stain) on the slides and confirm whether these smears have good quality enough for ICC before processing ICC. Alternatively, we can use pre-stained slides submitted for routine cytologic examination for subsequent ICC, if they are already diagnosed with lymphoma by a pathologist.
While generally unstained slides yield stronger immunocytochemical responses, the difference of the signal intensity is minimal between unstained and Romanowsky-stained (e.g. Wright-Giemsa, Diff Quik) slides. Furthermore, the difference of signal intensity does not affect the diagnosis of immunophenotyping2.
Air-dried cytology slides are acceptable for use in ICC as described above, but cell viability and antigen-antibody responses are decreased if the slides remain at room temperature for a long time. If you want to keep air-dried slides for use of ICC in your clinic, storage at 2°C to 8°C for up to 2 weeks before ICC does not appear to affect antigenicity. The slides should be put in a plastic slide box and then in a zip-lock plastic bag with desiccant. Samples need to equilibrate to room temperature before opening the bag to avoid cell rupture3.
Lymphoid cell phenotype can also be determined via flow cytometry (FC) and PCR for antigen receptor rearrangement (PARR). FC and PARR are also minimally invasive tests, but they are not routinely available domestically at present. Note that we are able to send slides overseas for PARR testing; however, turnaround time can be prohibitive in some cases. Therefore, ICC is a routinely available test without extra time and effort to the submitting veterinarian to identify the immunophenotype of lymphoid cells in dogs.
References:
1. Sapierzynski, R. Practical aspects of immunocytochemistry in canine lymphomas. Polish Journal of Veterinary Sciences, 13: 661-668, 2010
2. Raskin, R. E., Vickers, J., Ward, J. G. et al. Optimized immunocytochemistry using leukocyte and tissue markers on Romanowsky-stained slides from dogs and cats. Veterinary Clinical Pathology, 48: 88-97, 2019
3. Raskin, R. E., Meyer, D. J. Advanced Diagnostic Techniques. In: Canine and Feline Cytology, 3rd ed. (Wilson, L. ed.), pp. 453-494, Elsevier, St. Louis, 2016.