Sandra Bulla
An update:
Mast cell tumours (MCT) are the most common skin neoplasia in dogs, shown to occur with a frequency of approximately 20% of all canine skin tumours. Although it can occur in any breed, the prevalence is consistently reported in many studies from different countries to be higher in some breeds. Some examples of most affected dogs are Boxers, Labradors, Golden Retrievers and Bull Terriers (White et al., 2011, Warland and Dobson, 2013, Smiech et al., 2018).
MCTs are more often cutaneous, although the clinical presentation of the lesions can vary widely. They commonly present as a hairless, singular, raised, and erythematous solid lesion. However, they can also be haired, vary from firm to soft, and be pruritic. Some cases may present as irregular, raised masses. They can sometimes invade the subcutaneous tissue and be ulcerated.
A mast cell neoplasia can also originate from the subcutaneous tissue. In these cases, the disease was shown to have mostly a favourable prognosis, with high survival times and low rates of reoccurrence. Metastasis and reoccurrence were strongly predicted by mitotic index in histopathology. Presence of multinucleation and tissue infiltration by the neoplasia rather than well circumscribed masses also predicted survival (Thompson et al., 2011). Although the location of MCT can frequently be correctly assessed by palpation and observation of macroscopic features, cutaneous and subcutaneous lesions can sometimes be similar and difficult to differentiate clinically. Thus, the location can only be definitively determined by histopathological examination of an excised mass (de Nardi et al., 2022).
Diagnosis of MCTs is commonly achieved by routine cytology. The typical cytological features of these neoplastic cells are easily recognised in aspirates from these masses. They usually exfoliate readily, yielding highly cellular slides containing numerous round, individualised cells with round nucleus and moderate amounts of pale cytoplasm. The cells in well granulated MCT have numerous cytoplasmic thick purple granules. In poorly granulated ones, the granules are smaller and present in low numbers to almost non-existent. Variable numbers of eosinophils and reactive fibroblasts and variable amounts of collagen fibrils are also often present.
The prognosis of canine cutaneous MCT is highly variable and depends greatly on histopathologic grading. Currently, there are two systems that are used for predicting prognosis in the evaluation of biopsy samples. The Patnaik three-tier system (Patnaik et al, 1984) classifies the tumour in three grades, depending on tissue invasion, presence of cellular atypia, granularity, nuclear features, mitotic count, and multinucleation. Dogs with grade I and III MCTs present the highest and lowest life expectancy, respectively. The grade II is more unpredictable, and prognosis can vary from good to poor.
More recently, a more objective two-tier system was proposed (Kiupel et al., 2011), based on mitotic count, presence of multinucleation, bizarre nuclei, and karyomegaly. The high-grade MCTs have a worse prognosis than low-grade MCT. The current criteria for most pathologists and the recommendation by consensus of pathologists and oncologists are that both grading systems are applied concurrently to better classify the disease (Berlato et al., 2021, De Nardi et al., 2022). Additionally, in certain cases the use of other prognostic markers might be useful, especially for grade II or in subcutaneous MCT. The most used are mitotic index and staining for AgNOR, Ki67, and KIT. The presence of AgNOR and Ki67 are associated with significant decreased survival whereas the pattern of KIT expression can help determine if biologically aggressive behaviour is to be expected. Increased cytoplasmic KIT expression, opposed to membranous expression, is associated with an increased risk of local recurrence and lower overall survival (Kiupel et al., 2004).
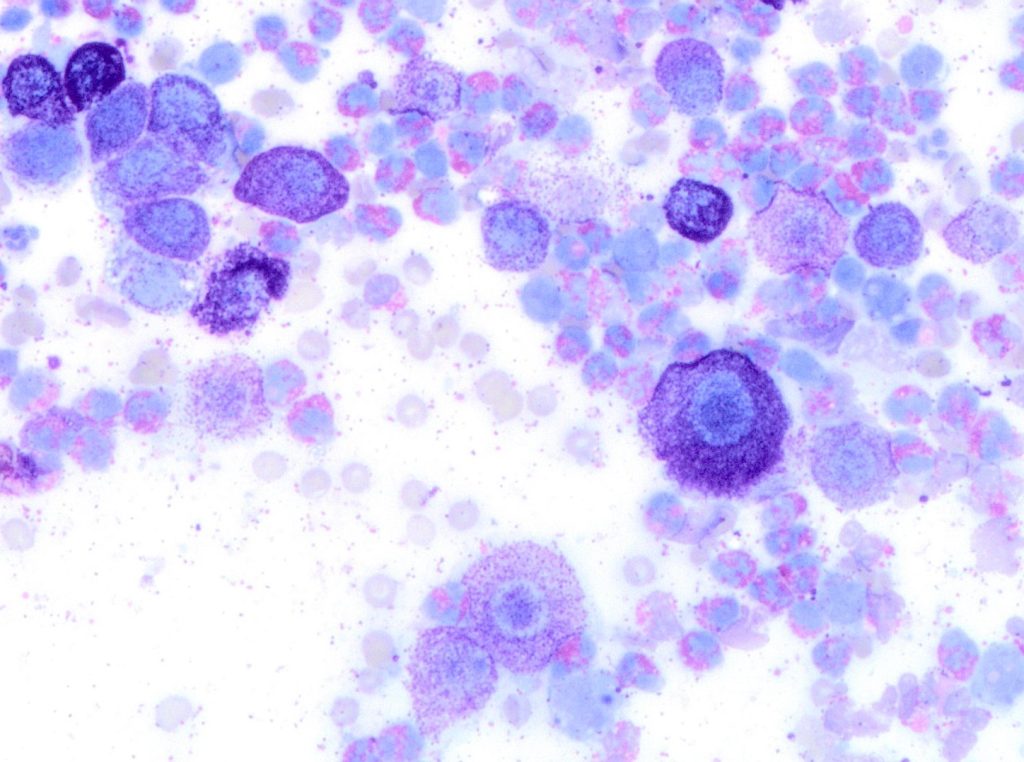
In the last decade, a few studies attempted to describe a system for cytologic preparations of MCTs samples that could predict clinical behaviour of the tumour. In 2014 and 2016, two studies applied the Kiupel grading system on cytology slides. Both focused on evaluating the presence of mitotic figures, multinucleated cells, bizarre nuclei, and karyomegaly. Histopathology was used as the gold standard for grading, and overall, both studies found good correlation between MCT grades determined cytologically versus histologically. Cytologic grading matched histologic grading in 94% of cases in both studies. However, there were important cytologic limitations, including fewer numbers of cells for evaluation and lower occurrence of mitotic figures on cytologic preparations compared with histologic preparations. (Scarpa et al 2014, Hergt et al 2016).
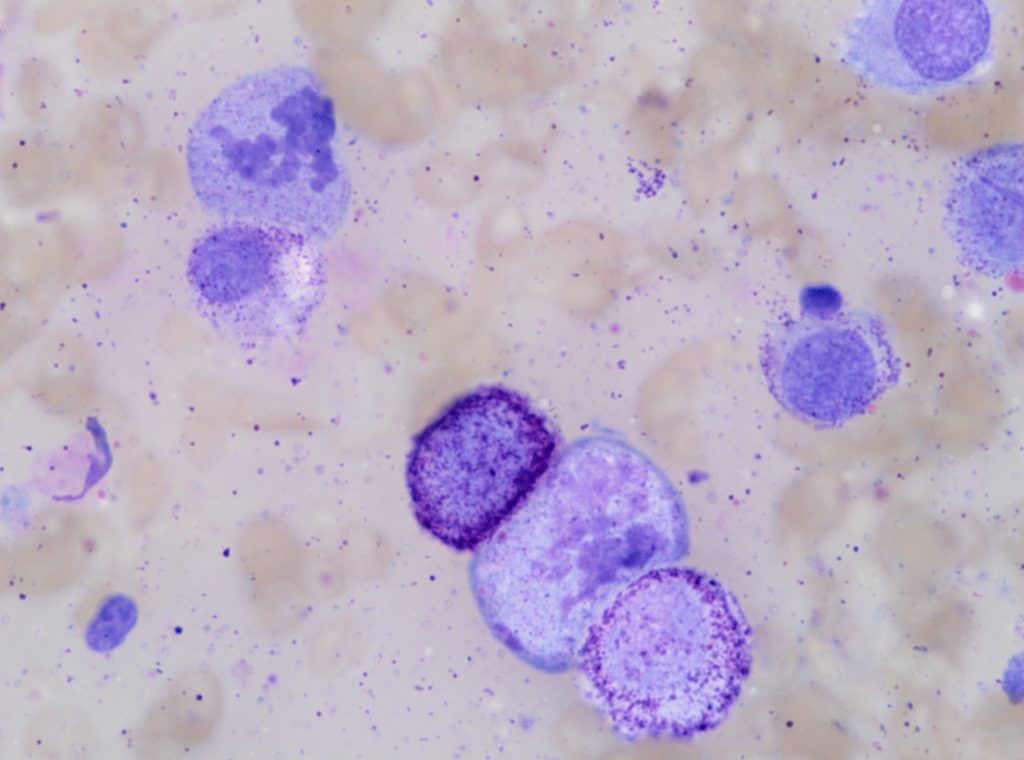
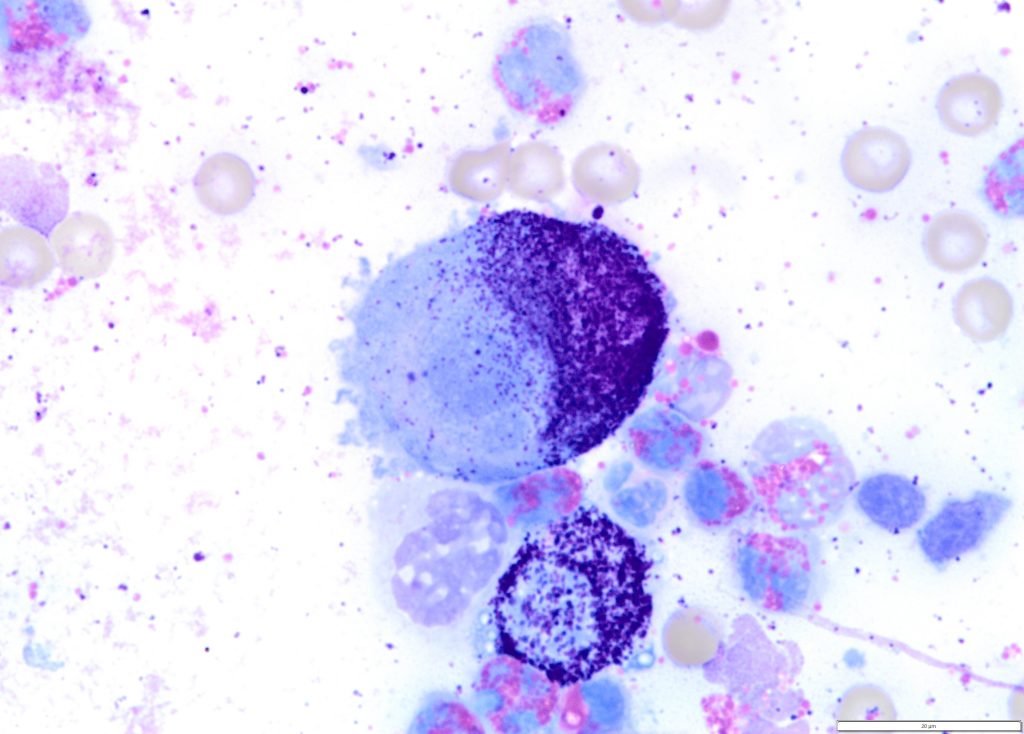
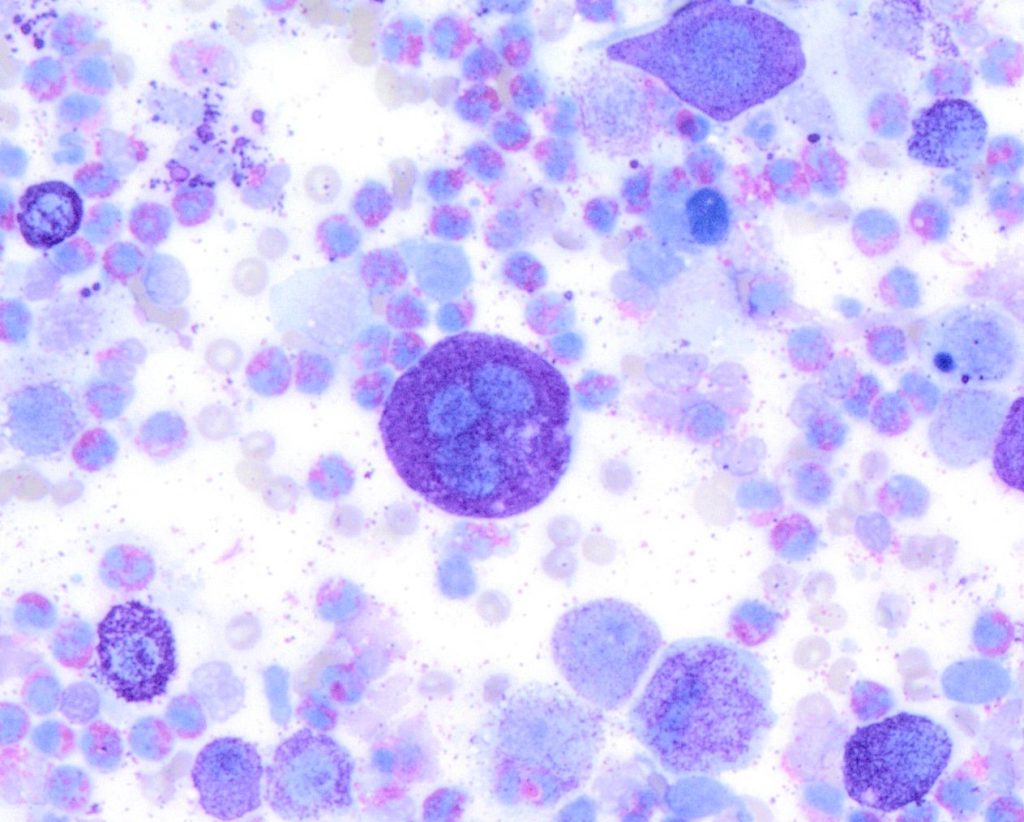
Figure 2. A high-grade cutaneous mast cell tumour in a dog. Presence of mitotic figures (A), anisokaryosis (B) and multinucleation (C).
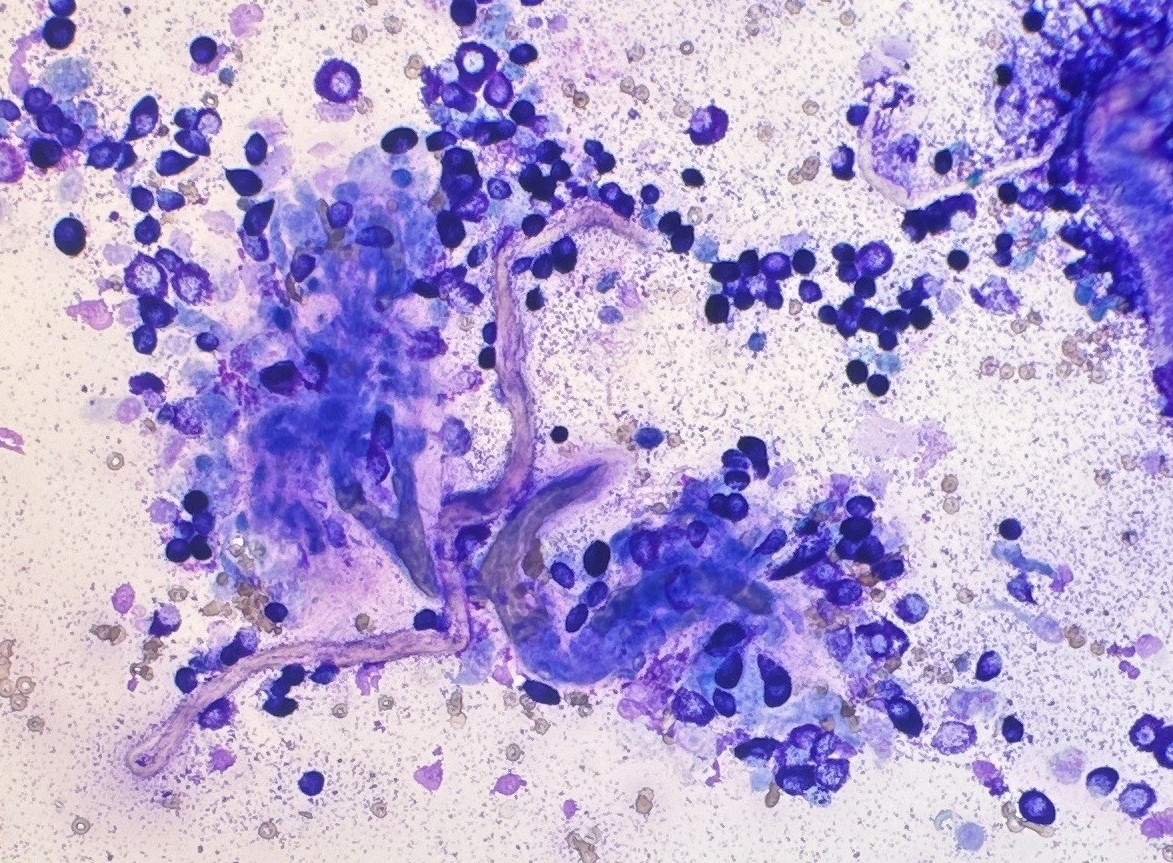
Then in 2016, a group of researchers from the University of Georgia, USA developed a new system that compared mast cell granulation as well as some nuclear features to the Kiupel histopathologic grade. The tumour was classified as high grade if it was (a) poorly granular (Figure 1) or (b) had at least 2 of the following 4 features: presence of any mitotic figures, anisokaryosis (>50%), bi/multinucleation, or nuclear pleomorphism (Figure 2). The cytologic grading scheme had 88% sensitivity and 94% specificity relative to histologic grading. Although there is a tendency to overestimate tumour grade compared with histopathology, as a screening test, this is preferable to underestimating tumour grade, which might result in aggressive tumours being undertreated (Camus et al., 2016). This system is currently the most widely used system by pathologist when trying to estimate MCT grade in cytology.
In an effort to improve the cytological grading published by Camus, a group from Brazil proposed the simultaneous assessment of the tumour microenvironment, with the incorporation of fibroblasts and/or collagen fibrils in cytologic grading scheme (Paes et al., 2022). They compared the Camus cytologic grading scheme with their modified system, patient one year survival rates, and the Kiupel histopathologic grade. Their work supported the results obtained by Camus and showed that fibroblasts and/or collagen fibrils are associated with lower grades of malignancy. The presence of granulated cells and high concentrations of fibroblast and/or collagen were what most correlated with high survival (Figure 3).
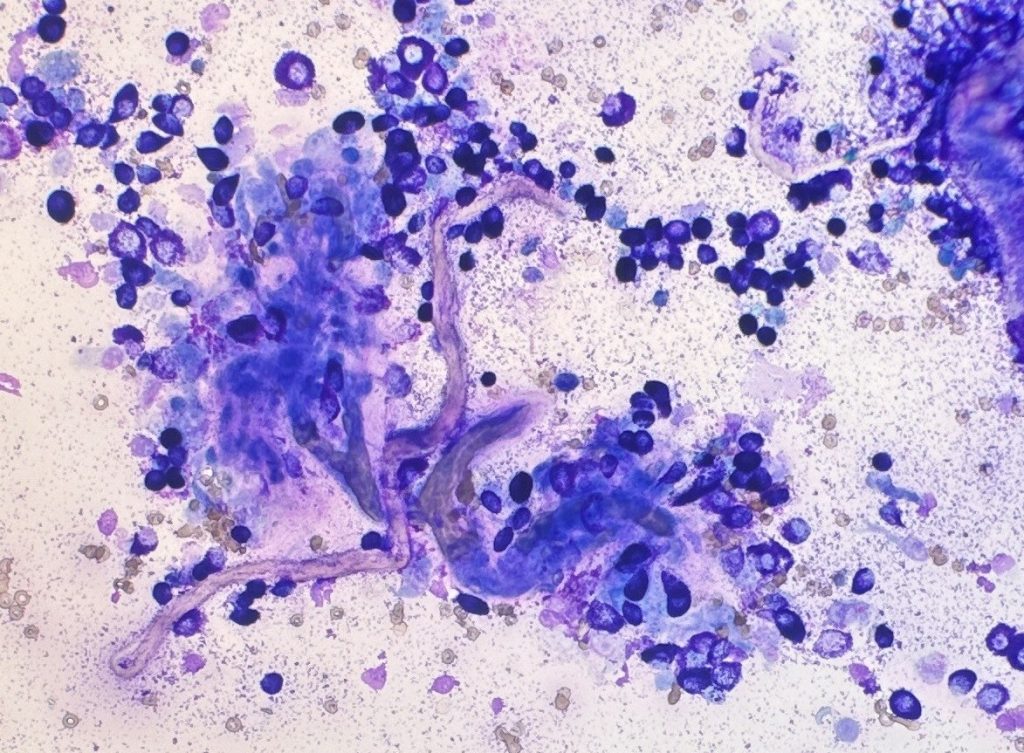
Due to the intrinsic features of cytology compared to histopathology evaluation, there are some limitations in cytologic grading of cutaneous MCTs. For examples, MCTs are known to be heterogeneous in the presence of mitosis and other nuclear features. Thus, the evaluation of nuclear morphology might be difficult since these features might not be easily found on the cytology slides. However, it is important to notice that cytologic grading is not supposed to replace histopathology, but rather be used as an initial screening test. This cytologic information might help the clinician formulate a patient care plan, guiding how aggressive to be in the patient’s workup. Additionally, a combination of cytology and histopathology grading might be what will provide the best prognostic information (Berlato et al., 2021, Krimer, 2002).
References:
· Berlato D, Bulman-Fleming J, Clifford CA, et al. Value, Limitations, and Recommendations for Grading of Canine Cutaneous Mast Cell Tumors: A Consensus of the Oncology-Pathology Working Group. Vet Pathol. 58:858-863, 2021.
· Camus MS, Priest HL, Koehler JW, et al. Cytologic Criteria for Mast Cell Tumor Grading in Dogs With Evaluation of Clinical Outcome. Vet Pathol. 53:1117-1123., 2016.
· Hergt F, von Bomhard W, Kent MS, Hirschberger J. Use of a 2-tier histologic grading system for canine cutaneous mast cell tumors on cytology specimens. Vet Clin Pathol. 45:477-483, 2016.
· Krimer PM. Inclusion of fibroblasts and collagen fibrils in the cytologic grading of canine cutaneous mast cell tumors. Vet Clin Pathol, 51:464-466, 2022.
· Kiupel M, Webster JD, Bailey S, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. 48:147-155, 2011
· Kiupel M, Webster JD, Kaneene JB, et al. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. 41:371-7, 2004.
· de Nardi AB, dos Santos Horta R, Fonseca-Alves CE, et al. Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors. Cells 11:618, 2022.
· Paes PRO, Horta RS, Luza LC, et al. Inclusion of fibroblasts and collagen fibrils in the cytologic grading of canine cutaneous mast cell tumors. Vet Clin Pathol. 51:339-348, 2022.
· Patnaik AK, Ehler WJ, MacEwan EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol. 21:469-474, 1984.
· Scarpa F, Sabattini S, Bettini G. Cytologic grading of canine cutaneous mast cell tumors. Vet Comp Oncol. 14:245-251, 2014.
· Śmiech A, Ślaska B, Łopuszyński W, et al. Epidemiological assessment of the risk of canine mast cell tumours based on the Kiupel two-grade malignancy classification. Acta Vet Scand. 60:70, 2018.
· Thompson JJ, Pearl DL, Yager JA, Best SJ, Coomber BL, Foster RA. Canine subcutaneous mast cell tumor: characterization and prognostic indices. Vet Pathol. 48:156-68, 2011.
· Warland J, Dobson J. Breed predispositions in canine mast cell tumour: a single centre experience in the United Kingdom. Vet J. 197:496-8, 2013.
· White CR, Hohenhaus AE, Kelsey J, Procter-Gray E. Cutaneous MCTs: associations with spay/neuter status, breed, body size, and phylogenetic cluster. J Am Anim Hosp Assoc. 47:210-6, 2011.