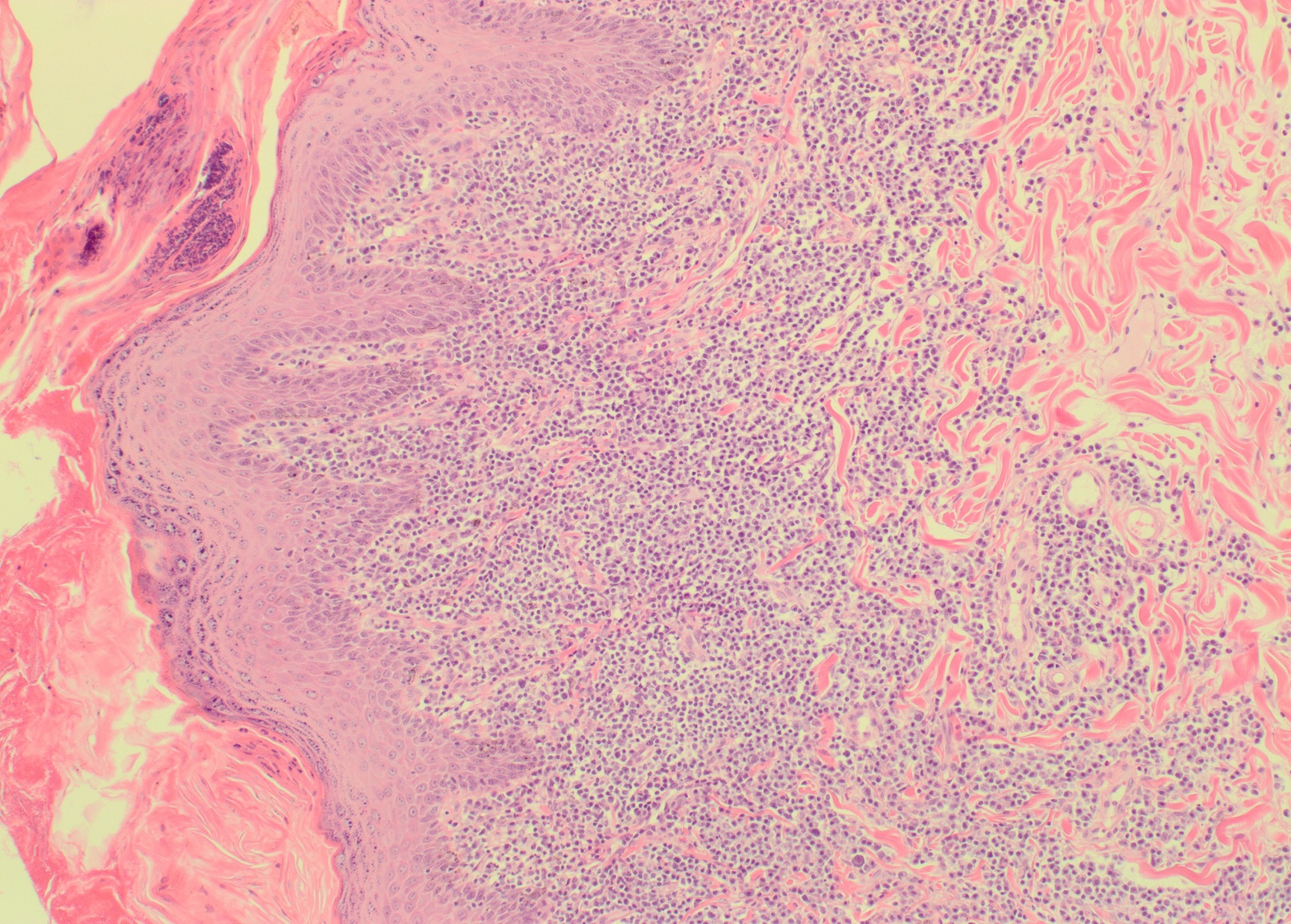
Species: All
Specimen: Fixed tissue (1:10 tissue:formalin)
Container: Plain sample pottle
Collection protocol:
Remember to provide as many clinical details as possible on your submission form. A picture tells a thousand words: if you can, send photos – digital photos can be emailed to your pathologist (usually firstname.lastname@awanuigroup.co.nz). Try to use a program that can reduce the size of your image files preferably to under 1MB. Alternatively, you can provide images on a CD or upload them to a file-sharing site (e.g. Dropbox). Pathologists will view these photos with the case slides and history provided on the submission form.
Special handling/shipping requirements:
Tissues in formalin should be submitted in separate sealed plastic bags from any specimens for cytology. Formalin fumes cause artefact in the cytology specimens that reduces staining quality to a level that is often insufficient for diagnosis. Specimens should be shipped in leak-proof containers and labelled appropriately (refer to How to package a sample).
Please be very careful not to put freshly removed tissue into narrow-necked containers. Fixed tissue becomes quite firm and warps in fixation; whilst you may have been able to squeeze it into a container, it can then conform to the shape of the container (losing its anatomic orientation) and may be impossible for us to retrieve it without cutting it or breaking the container.
The rule of thumb for tissue:fluid volume is 1:10. It is important to realise that formalin penetrates tissue slowly and might only penetrate 0.5 cm into a tissue block. This requires that the tissues placed in formalin be kept as thin as possible; having slices of only 1 cm in one dimension is optimal. If you cannot see a gross lesion, submission of a big chunk of tissue is false economy. In this situation, the centre will autolyse and it might be that only the outer, fixed part of the tissue will be trimmed anyway.
The standard all purpose fixative is 10% buffered formalin. This is made by adding 9 volumes of water to one volume of commercial formalin (available as 40% formaldehyde) and buffering it to pH 7. Formalin that is incorrectly buffered has a deleterious affect on nuclear staining and causes yellow-brown deposits to form in the tissues (acid haematin). Neutralised (buffered) formalin (NBF) is easily made by adding 5 g of limestone chips to every 2L of 10% solution. Otherwise, the following formula may be useful:
100 mL Formalin (40% commercial) + 900 mL distilled water + 6.5 g Na2HPO4 + 4.0 g NaH2PO4.H2O
Formalin vapour is an irritant and potentially carcinogenic. It is proven to cause dermatitis in susceptible individuals. Formalin should only be handled in well-ventilated areas and care taken at all times to avoid direct contact with the skin and mucous membranes; gloves are strongly recommended.
Formalin fixation makes most pathogens inert once complete, with the known exception of prion proteins.